During a series of informational sessions, EPA has broken down its management standards for hazardous waste pharmaceuticals and amendment for nicotine.
The U.S. Environmental Protection Agency (EPA) has finalized its standards for managing hazardous waste pharmaceuticals. The updated standards, “Management Standards for Hazardous Waste Pharmaceuticals and Amendment to the Hazardous Waste Listing for Nicotine,” aim to reduce the cost and compliance burden for the healthcare sector and ensure the safe management of hazardous waste pharmaceuticals, according to the agency.
Additionally, these changes aim to provide regulatory certainty and national consistency on how the Resource Conservation and Recovery Act (RCRA) applies to the reverse distribution of prescription pharmaceuticals. With this rule, EPA is also taking a regulatory approach to the disposal of Food and Drug Administration (FDA)-approved, over-the-counter nicotine replacement therapies (patches, gums and lozenges), which will no longer be considered hazardous waste when discarded.
During a series of webinars addressing the finalized standards, Kristin Fitzgerald, an environmental protection specialist with EPA’s Office of Resource Conservation and Recovery, explained the rule package, which is nearly 500 pages, should be looked at as three rules rolled into one. The final rule was published in the Federal Register on February 22, 2019.
The first part of the rule, Subpart P, which is bulk of the rule, creates more flexible regulations for the healthcare sector, prohibits sewer disposal of hazardous waste and eliminates some regulations that also fall under FDA.
The second part of the rule is to provide regulatory and national consistency on how RCRA applies to reverse distribution and reverse logistics.
And the third part of the rule is an amendment to Part 261, the amendment of nicotine for P075 hazardous waste listings.
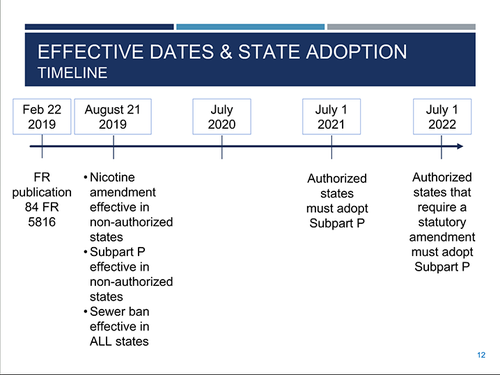
“Even though the rule is out and published, you can’t implement the rule yet,” explained Fitzgerald. “It won’t be effective in authorized states until each state adopts it.”
Currently, nicotine is listed as an acute hazardous waste under P075. This final rule amends the P075 listing for nicotine for FDA-approved, over-the-counter nicotine replacement therapies. That means nicotine gums and lozenges can be discarded as nonhazardous waste after the rule takes effect.
The amendment to the nicotine listing is effective six months after application in the Federal Register, so August 2019 federally in nonauthorized states and U.S. territories. Once it’s in effect, the amendment for P075 listings is applied to any generator of over-the-counter nicotine products. However, in authorized states, the amendment to the nicotine listing is only effective after a state adopts it.

“The amendment to the nicotine listing is considered less stringent than the current program,” noted Fitzgerald. “Therefore, authorized states are not required to adopt the amendment, and they have no deadline to adopt the amendment. We do expect most states will pick it up, but they are not required to.”
In general, nicotine will, however, continue to be listed as a hazardous waste under code P075. According to Fitzgerald, that means other unused formulations of nicotine—e-cigarettes and cartridges, for example—will still be considered P075 when discarded. In addition, there are legacy pesticides containing nicotine that will still fall under the P075 hazardous waste criteria.

Subpart P of the final rule is effective six months after publication in the Federal Register—also August 2019—but only in nonauthorized states and in U.S. territories. As with nicotine, Subpart P is only effective in authorized states after states adopt it.
However, Subpart P rules are more stringent, and states are required to adopt them. Authorized states will have until July 1, 2021, to adopt Subpart P, unless they have a statutory amendment, then they would have until July 1, 2022.

The following are not subject to the RCRA Subpart P regulation:
Pharmaceuticals that are not solid waste because they are legitimately used/reused or reclaimed.
Over-the-counter pharmaceuticals, dietary supplements or homeopathic drugs that are not solid waste because they have a reasonable expectation of being legitimately used/reused or reclaimed.
Recalled pharmaceuticals, which do become subject to Subpart P when the decision is made to discard.
Pharmaceuticals under preservation order or during an investigation or judicial proceeding. (They do become subject to Subpart P when the decision is made to discard.)
Investigational new drugs, which do become subject to Subpart P when the decision is made to discard.
Household waste pharmaceuticals.

When it comes to the sewer prohibition of the rule, there are no exceptions and the regulations are even more stringent. The sewer prohibition will be effective in all states six months after published in the Federal Register, regardless of whether the state is authorized or the final rule is adopted in the state. The effective date of the sewer prohibition aspect of the rule is August 2019 for all the states.
According to EPA, hazardous waste pharmaceuticals may not be sewered, meaning no drain disposal or flushing. The sewer prohibition applies to all healthcare facilities and all reverse distributors. Hazardous wastes that are Drug Enforcement Agency-controlled substances are also subject to the sewer prohibition.
As for the distinction between reverse distribution versus reverse logistics, Laura Stanley, an economist with EPA’s Office of Resource Conservation and Recovery, explained that reverse distribution refers to the process in which prescription pharmaceuticals are sent to a reverse distributor; for instance, a healthcare facility, where they are evaluated to see if they will receive manufactured credit. In this instance, no redistribution occurs, so the final rule maintains that prescription pharmaceuticals moving through reverse distribution are to be treated as hazardous waste at the healthcare facilities.
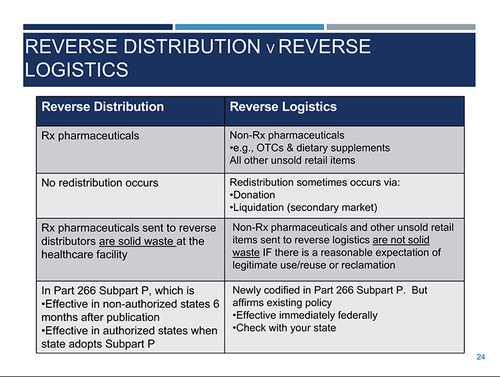
Reverse logistics refers to the process where non-prescription pharmaceuticals—anything over the counter, supplements and other retail items—are sent to a reverse logistics center and evaluated to see if they will be reused or reclaimed. These materials will not be considered waste at the healthcare facility or the retail facility if they have a reasonable expectation of being reused or reclaimed, noted Stanley. If there is no expectation of reuse or reclamation, pharmaceuticals must be disposed of as hazardous waste by the healthcare or retail facility.
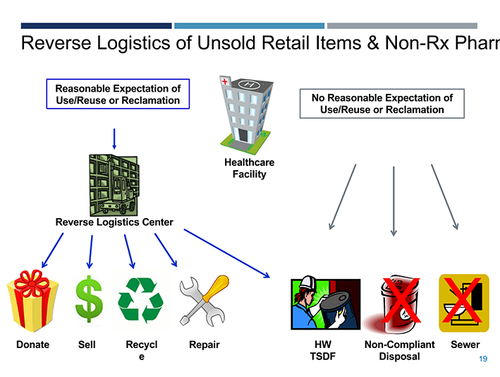
The finalized healthcare facility standards are meant to keep more pharmaceuticals out of landfills, according to EPA. Over the next six months, facilities and generators can check with their state to see where they fall under the regulations.
About the Author(s)
You May Also Like




